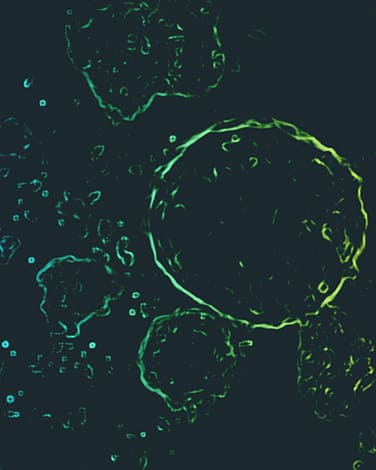
GAIA
NK-like cell
platform
A breakthrough in
eliminating solid tumors
A major limitation of adoptive cell immunotherapy is its insufficient therapeutic efficacy, even with the use of genetically modified chimeric antigen receptor (CAR)-T and natural killer (NK) cells.
Our platform enables the production of NK-like cells that effectively eliminate solid tumors, without any genetic modification.
GAIA's antibody-anchoring
and activating peptide
A peptide-based platform
to generate GAIA NK-like cells
targeting a variety of tumors
GAAAP enables the generation of GAIA NK-like cells that maintain their active state in vivo and specifically target cancer cell-expressed proteins.
Core Technology IINews
-
News
GAIA BioMedicine Initiates Development of Proprietary Cell Processing Center (CPC) at “f-labs Kyushu University Hospital” – Paving the Way for Commercial Production of Innovative Cancer Therapies –
More -
News
GAIA-102 interim data in pediatric neuroblastoma to be presented at the 67th Annual Meeting of the Japanese Society of Pediatric Hematology/Oncology
More -
News
Interim report on physician-initiated clinical trial of GAIA-102 for peritoneal dissemination received Excellent Presentation Award at the 97th Annual Meeting of the Japanese Gastric Cancer Association
More -
News
Prof. Yoshikazu Yonemitsu will present at the 31st Annual Meeting of Japan Society of Gene and Cell Therapy
More -
News
The progress report on the safety and clinical efficacy of GAIA-102 against pediatric solid malignant tumors will be presented at the 66th Annual Meeting of the Japanese Society of Pediatric Hematology/Oncology
More